Colon and Rectal Surgery Fellowship
The Division of Colon and Rectal Surgery offers ACGME-accredited training in colon and rectal surgery.

2024-2025 Fellow, Tyler Collins with program faculty at the 2025 fellowship graduation dinner.
Our fellowship program is one year in duration and is based at Millard Fillmore Suburban Hospital, supplemented by opportunities to operate at Buffalo General Medical Center, Sisters of Charity Hospital, Kenmore Mercy Hospital and Mercy Hospital of Buffalo.
During the year, the fellow is exposed to and expected to become proficient at office practice, outpatient procedures, inpatient anorectal surgery, major colonic surgery, laparoscopic colon surgery, robotic colon surgery, diagnostic and therapeutic colon and rectal surgery, and stomal care.
Successful completion of this year qualifies the fellow to take the certifying examination offered by the American Board of Colon and Rectal Surgery.
The program accepts one fellow per year, and participates with the National Resident Matching Program (NRMP). Prospective fellows are usually invited for an interview in August or September of the preceding year.
Applicants must have completed full general surgical training in a recognized training program (ACGME) and be eligible for the qualifying examination of the American Board of Surgery.
Colorectal Balloon Tube Clinical Trial
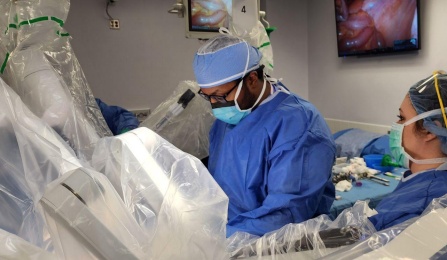
Former colorectal surgery fellow, Dr. Jaafar Elnagar performing the first procedure in the U.S. for the clinical trial.
Former colorectal surgery fellow, Dr. Jaafar Elnagar with Jeffrey Visco, MD
Our colorectal surgery attendings are participating in a clinical trial being done at Millard Fillmore Suburban Hospital (MFSH) utilizing a device that revolutionizes colorectal cancer surgery treatment, recovery, and quality of life for patients.
COLO-BT is a medical device used to avoid the need for an ileostomy. An ileostomy is a surgical procedure that attaches part of the small intestine to an opening, or stoma, in the abdominal wall so waste can exit when the colon is not functioning properly. Most patients need a temporary ileostomy for 3-6 months following colorectal cancer surgery to allow the intestines to heal. Then the ileostomy is reversed requiring a second surgery. COLO-BT completely removes the need for an ileostomy and the second reversal surgery. The device significantly reduces the risk of complications associated with colorectal cancer surgery and those commonly developed afterward.
The COLO-BT study is set to allow 256 patients who meet specific criteria to participate in the clinical trial. Baylor and Penn State are also conducting the trial, however, UBMD surgeons at MFSH are serving as the lead investigators in the clinical study.

Colorectal surgery attendings participating in new colorectal cancer medical device clinical trial.
Contact
Training Program Administrator
Sydney Ruger, M.Ed
Surgical Fellowship Administrator
Department of Surgery
Buffalo General Medical Center, 100 High Street, Buffalo, NY 14203
Phone: 716-859-4207
Email: slrojek@buffalo.edu
